A Brief History of Lyrica
Lyrica was initially approved by the FDA in 2004. Following approval for further indications in 2007 and again in 2012, Lyrica’s patent was finally set to expire at the end of 2018. Since Lyrica is routinely seen as one of the higher spend medications in Workers’ Compensation, the industry was eagerly awaiting the calendar to turn to 2019 to see generics enter the market.
However, Pfizer continued its protection of the patent and received one last extension in 2018 related to pediatric exclusivity. This would prevent generic competition until the end of June 2019. Finally, after a slight delay, on July 19, the FDA approved applications of multiple competing manufacturers for generic pregabalin on all strengths of immediate-release Lyrica. Coincidentally, Pfizer received approval for Lyrica CR, an extended-release formulation of pregabalin, in October 2017. By adding this extended-release option to its portfolio, Pfizer was setting itself up to maintain brand name Lyrica usage for several more years.
Generic Drugs Save Money
A generic medication is approved via an abbreviated new drug application. Applicants do not need to repeat the animal and human studies that were required by the brand manufacturer in order to demonstrate safety and efficacy. Instead, a generic manufacturer must simply demonstrate that the generic medication performs the same in the body as the brand name drug in regards to dosage, safety, effectiveness, strength, stability, and quality.
A reduction in research costs means that a generic medication can be sold at substantial discounts compared to the brand medication, despite having the same therapeutic effect. To put that into real dollars, the IMS Health Institute reports that generic drugs saved the United States healthcare system $1.67 trillion from 2007 to 2016.
Savings Continue to Increase
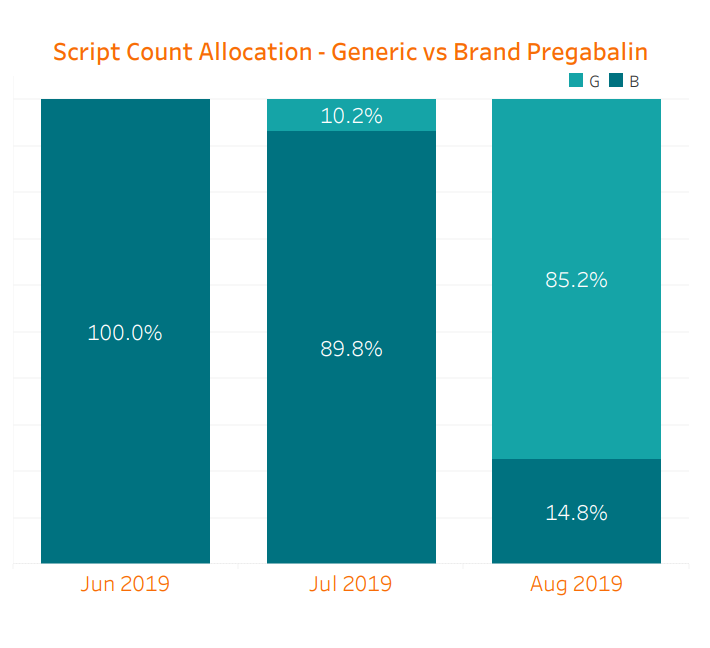
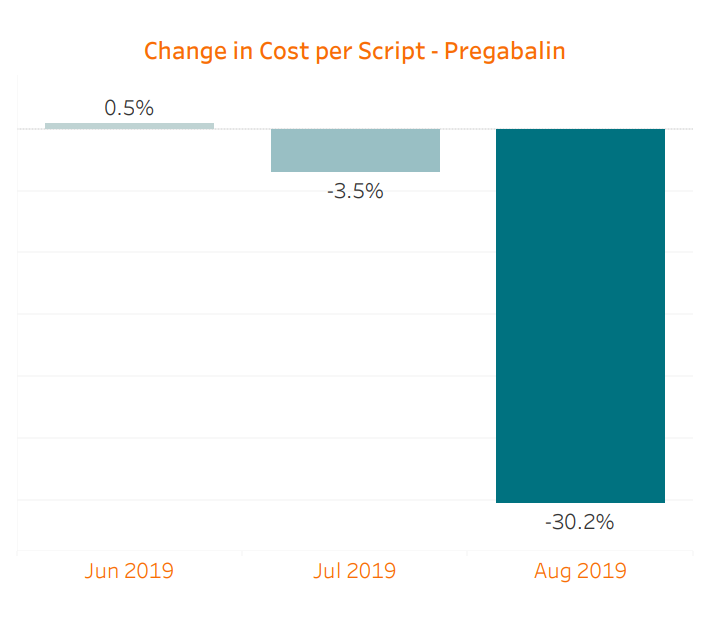
We are already seeing the benefit of generic competition starting to manifest. Because the generic was not approved until the latter half of the month and the supply was not readily available then, the impact in July was limited. When combining prescriptions of brand Lyrica, Lyrica CR, and generic pregabalin relative to June, July saw a slight 3.5% decrease in cost per script. However, in August, myMatrixx data shows an 85% generic utilization of pregabalin across our book of business, along with a further cost reduction of 30.2% per script. Total spend on Lyrica decreased an astonishing 54% compared with July despite a comparable prescription count. Considering the sizable historical spend on Lyrica, these changes will lead to significant savings opportunities for our clients. It is worth noting that brand-only Lyrica CR has made up less than 1% of all of these prescriptions.
What’s Next?
We have all been waiting for this for a long time. However, a generic drug does not provide much benefit if it is not utilized. myMatrixx will continue to monitor the impact of generic Lyrica as it relates to utilization and spend. For any questions you may have, please reach out to your Clinical Account Executive or Clinical@myMatrixx.com