Senior Clinical Account Executive
Hepatitis C, a viral infection of the liver caused by the hepatitis C virus (HCV), can be spread through contaminated blood and other body fluids. The infection can range in duration from a few weeks (acute) to a life-long illness (chronic). Between 75% and 85% of the people who become infected with hepatitis C will develop the chronic form1, CHC, which now affects more than 3 million people in the U.S.2 CHC may lead to chronic liver diseases, including cirrhosis and liver cancer. With the 2011 arrival of newer therapies to treat HCV (specifically direct-acting antiviral therapies, or DAAs), alcohol-related liver disease now has surpassed HCV as the leading cause of liver transplantation in the U.S., and HCV as an indication for liver transplantation is expected to continue its decline.3
I have received a request for a medication to treat hepatitis C. Will you please tell me why hepatitis C drugs might be needed to treat an occupational injury?
While we don’t see a lot of hepatitis C patients in workers’ compensation, it may be appropriate for claims under certain situations. Occupational exposure to hepatitis C could result from needlesticks in some injured worker populations, such as healthcare personnel, first responders and other municipal workers. The risk of HCV infection following a needlestick or sharps exposure to HCV positive blood is approximately 0.1%.1 Injured patients who received blood or organs from an HCV-positive donor also could be infected. Unlike for hepatitis A and hepatitis B, no vaccine currently is available for hepatitis C.1 Further, not enough evidence is available to support the effectiveness of post exposure prophylaxis, or PEP, after potentially being exposed to HCV.4
Recommendations from the Centers for Disease Control and Prevention (CDC)5:
- PEP is not recommended for hepatitis C.
- PEP following an occupational needlestick does include antiviral drugs for human immunodeficiency virus (HIV) and vaccination for hepatitis B, however.
-
Pre-existing chronic infection:
- The occupationally exposed worker should be tested within 48 hours of exposure to determine the presence of antibodies to the hepatitis C virus (anti-HCV).
- Anti-HCV will be present if the exposed worker has previously been infected with hepatitis C. If positive, further testing and referral to care for pre-existing CHC infection may be needed.
-
Infection as a result of the occupational exposure:
- Those who test negative within the first 48 hours should be tested for HCV RNA three or more weeks after exposure to determine whether HCV then exists in the exposed worker’s bloodstream, with referral for care for a positive test as a result of the occupational exposure.6
- Patients may spontaneously clear an acute infection up to six months after exposure. Therefore, all exposed workers who test positive in less than six months should be tested again at least six months after exposure to determine existing infection status.
Are the newer hepatitis C drugs much different from the older ones and why are they so expensive?
In addition to the cost of treatment, the choice of medication treatment protocol should take into account the genetic makeup, which is known as the “genotype”, of the virus. Hepatitis C has seven recognized viral genotypes1. Knowing the genotype is important to determine the most appropriate medications once a person has been diagnosed with CHC. In the U.S., about 70% of CHC cases are genotype 11, which has a lower response rate to older hepatitis drugs like ribavirin and injectable pegylated interferon, than other genotypes.7
DAAs, the newer treatment options for CHC, are available in oral form, so they are more convenient to use. They are much more expensive than earlier drugs; but they produce substantially higher cure rates than the older medications, more than 90% for many patients in as little as eight weeks. Before DAAs were introduced, the success rate for previous HCV therapies was only about 41% and severe side effects often were associated with using them.8
Curing an exposed worker of the HCV infection prevents chronic liver disease and possible liver cancer or transplantation. In addition, DAA medications are effective for most patients without requiring multiple courses of therapy. Even at their high initial cost compared to other drugs, they typically cost much less than managing liver cancer or undergoing a transplant along with their corresponding follow-up treatments.
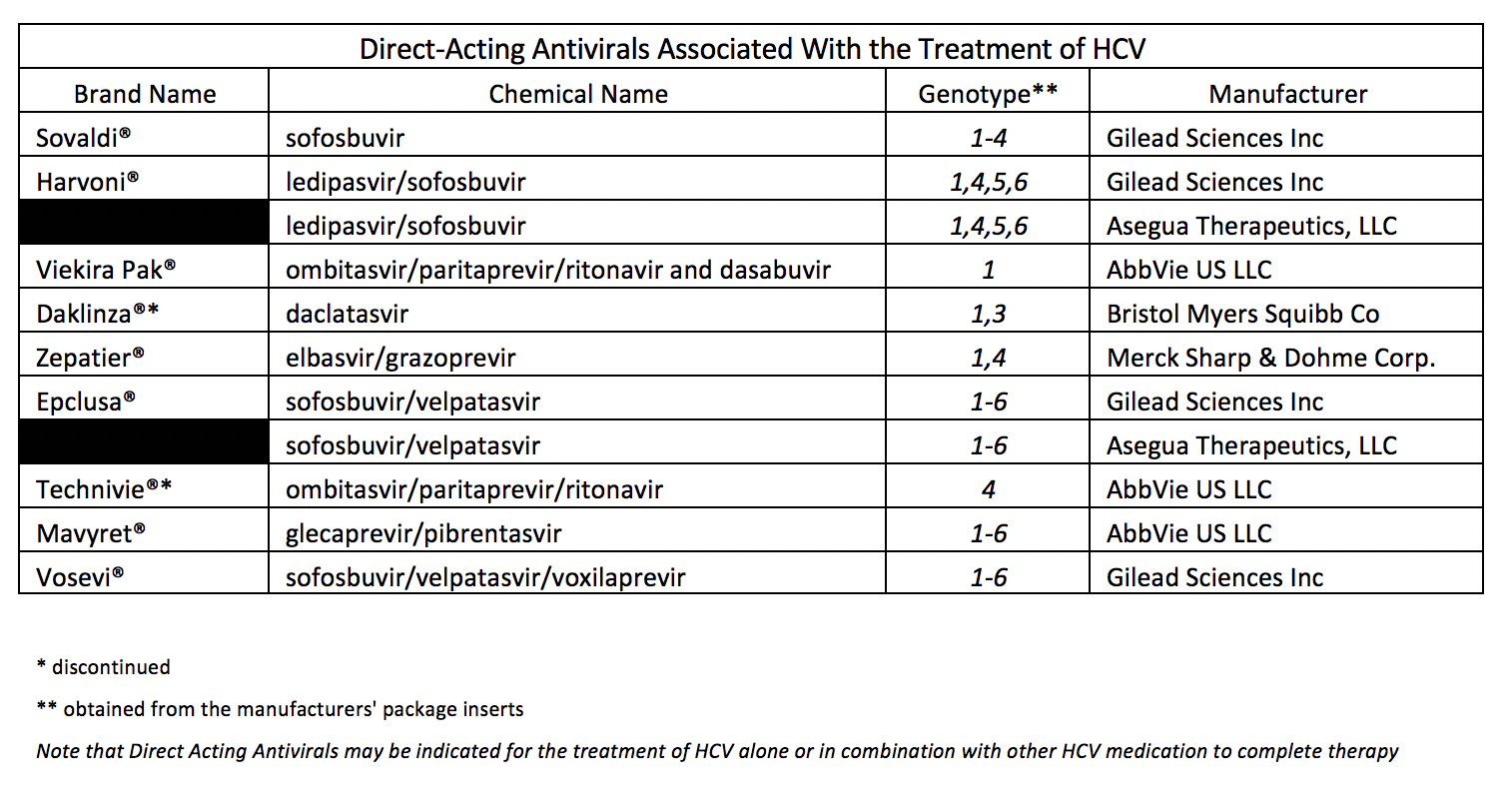
I have heard some of the newer hepatitis C drugs have generics. Can you provide details?
Yes. Authorized generics to Harvoni® (ledipasvir 90mg/sofosbuvir 400mg tablets) and Epclusa® (sofosbuvir 400mg/velpatasvir 100mg tablets) became available early 2019. Gilead Sciences, Inc., the manufacturer of both medications, made them accessible through a newly created subsidiary, Asegua Therapeutics LLC. The Average Wholesale Prices (AWP) for the generics are significantly less than the brand name medications.
Do DAAs have any drawbacks?
Treatment for CHC is evolving quickly, and so are treatment guidelines. The promising news is the DAAs that cure hepatitis C offer hope of eliminating it in the near future. Unfortunately, however, data from the CDC indicate the number of new HCV infections is on the rise. From 2010 to 2015 the number of acute hepatitis C cases reported to the CDC nearly tripled – mainly from increased injection-drug abuse. Improved case detection contributed to this increase as well, but to a much lesser degree. 9 Symptoms are often mild and vague in acute cases, making diagnoses difficult.
Not every patient is cured after one course of DAA treatment. A small percentage fail the initial therapy and need another round, usually with a different set of drugs. Hepatitis C will recur for some treated patients and others may be re-infected after CHC has been cured.
Another major concern related to the development of new HCV therapies is the emergence of resistance to DAA drugs. Drug resistance occurs when the hepatitis C virus no longer responds to treatment. This challenge to chronic HCV treatment is developing rapidly and it already has shown clinical impact on available DAA regimens. Drug-resistant viruses most frequently develop when drug doses are below therapeutic levels. However, they can also emerge when DAA therapy fails.10,11
CONCLUSION
As stated previously, within workers’ compensation, the prevalence of hepatitis C is rare. However, the higher cost of new drug therapies can make a significant impact on workers’ compensation payers even if only used by a small portion of their injured worker population. Curing the infection is important, though, to prevent progressive liver damage that can result in debilitating and costly outcomes.
- Centers for Disease Control and Prevention. Hepatitis C questions and answers for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm#section1. Last updated April 30, 2018. Accessed Dec. 7, 2018.
- U.S. Department of Health and Human Services. Office of Population Affairs. Hepatitis C. https://www.hhs.gov/opa/reproductive-health/fact-sheets/sexually-transmitted-diseases/hepatitis-c/index.html. Last reviewed April 10, 2018. Accessed Dec. 7, 2018.
- Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16(8):1356-1358. doi: 10.1016/j.cgh.2017.11.045.
- Hughes HY, Henderson DK. Postexposure prophylaxis after hepatitis C occupational exposure in the interferon-free era. Curr Opin Infect Dis. 2016;29(4):373-380. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5527758/. Accessed Dec. 7, 2018.
- Centers for Disease Control and Prevention. Information for healthcare personnel potentially exposed to hepatitis C virus (HCV). https://www.cdc.gov/hepatitis/pdfs/testing-followup-exposed-hc-personnel.pdf. April 2018. Accessed Dec.7, 2018.
- American Association for the Study of Liver Diseases and Infectious Diseases Society of America. HCV testing and linkage to care. https://www.hcvguidelines.org/evaluate/testing-and-linkage. Last updated May 24, 2018. Accessed Dec. 7, 2018.
- NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19(3):1-46. https://consensus.nih.gov/2002/2002HepatitisC2002116html.htm. Archived. Accessed Dec. 7, 2018.
- Pharmaceutical Research and Manufacturers of America. Twenty-five years of progress against hepatitis C: setbacks and stepping stones. http://phrma-docs.phrma.org/sites/default/files/pdf/Hep-C-Report-2014-Stepping-Stones.pdf. December 2014. Accessed Dec. 7, 2018.
- Centers for Disease Control and Prevention. Viral hepatitis. https://www.cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm. Last updated June 19, 2017. Accessed Dec. 7, 2018.
- American Association for the Study of Liver Diseases and Infectious Diseases Society of America. HCV resistance primer. https://www.hcvguidelines.org/evaluate/resistance. Last updated May 24, 2018. Accessed Dec. 7, 2018.
- Downward E. Drug resistance. HepatitisC.net. https://hepatitisc.net/treatment/drug-resistance/. Last reviewed March 2018. Accessed Dec. 7, 2018.
To learn more about our Clinical programs, email Clinical@myMatrixx.com.